Abstract
Introduction
Among available therapies, allogeneic stem cell transplantation (allo-SCT) is considered the only curative option in patients with myelodysplastic syndromes (MDS). Recently, two scores were developed to predict outcome of MDS patients following allo-SCT: the transplantation risk index (TRI) by GITMO and the prognostic score (PS) by CIBMTR. Our aim was to validate these scores using the European Society for Blood and Marrow Transplantation (EBMT) cohort and, where applicable, to optimize them using a transplant-specific approach.
Methods
In total, we included 1059 patients with MDS who underwent an allo-SCT from a HLA-identical sibling or matched/-mismatched unrelated donor (MUD) and were reported to the EBMT registry between 2000 and 2014. Patients were screened for eligibility according to factors used for each score. The TRI dataset consisted of 480 patients with full data on: age (< 50 or ≥ 50 years), IPSS-R (low, intermediate, high, very high), monosomal karyotype (MK; yes or no) and refractoriness to chemotherapy (yes or no). Karnofsky score (< 90 or 90 to 100%) was used as a proxy for an unavailable HCT comorbidity index. Patients were classified according to TRI scores: low (n = 39), intermediate (n = 219), high (n = 108) and very high (n = 114). A total of 667 patients met the following criteria of the PS: age (18 to 29, 30 to 49 or ≥ 50 years), Karnofsky score (90 to 100 or < 90%), cytogenetics (very good/good/intermediate, poor/very poor or MK), blood blasts (> 3 or ≤ 3%) and platelets before allo-SCT (> 50 or ≤ 50 x 109/L). The PS risk groups were: low (n = 24), intermediate (n = 330), high (n = 286) and very high (n = 27). We validated each score by investigating 3-year overall survival (OS) probabilites with the use of the Kaplan-Meier method. Cox regression and hazard ratios (HR) were used for optimization and scores were compared using the concordance index.
Results
Three-year OS (95% confidence interval) of a low TRI was 57.4% (41.5 to 73.3), intermediate 44.4% (37.5 to 51.3), high 37.8% (28.0 to 47.6) and a very high TRI was 24.4% (16.0 to 32.8; p = 0.002). Considering PS, 3-year OS of the low-risk group was 69.2% (50.0 to 88.4), intermediate 55.2% (49.7 to 60.7), high 39.5% (33.6 to 45.4) and of the very high-risk group 24.0% (7.1 to 40.9; p < 0.001).
The median age of the EBMT cohort (n = 1059) was 57 years (range, 18 to 75). Median follow-up was 53.9 months (50.3 to 57.6). Seven independent predictors of OS (HR) were identified in the multivariable analysis: age ≥ 50 years (1.76), Karnofsky score < 90% (1.72), MUD (1.38), very poor cytogenetics or MK (1.62), blood blasts > 1% (1.42), platelet count ≤ 25 (1.45), and a positive cytomegalovirus (CMV) status of the recipient at allo-SCT (1.36).
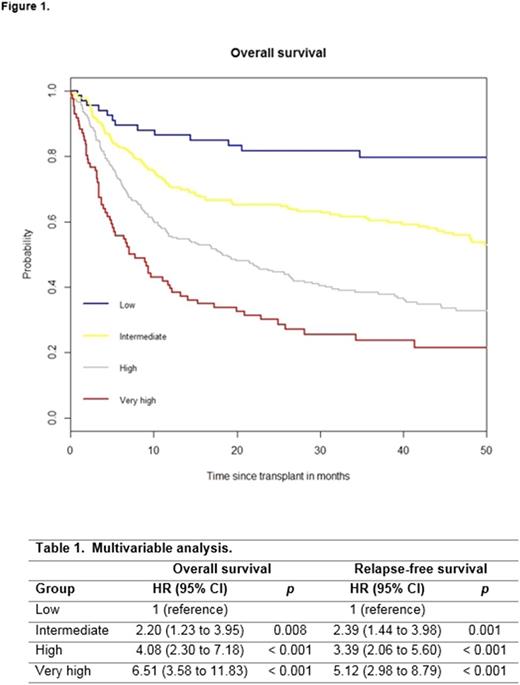
On the basis of full data on all seven predictors (n = 649) and a HR > 1.70, a weighted score of 2 was assigned to older age (≥ 50 years) and Karnofsky score < 90%, whereas other factors were assigned a score of 0 or 1 based on an HR < 1.30 and 1.30 to 1.70. The overall score ranged from 0 to 9. With respect to TRI and PS, we created a four-category system: low (score of 0 to 1, n = 69), intermediate (score of 2 to 3, n = 228), high (score of 4 to 5, n = 266), and very high (score of ≥ 6, n = 86). The 3-year OS probabilites were 79.7% (69.7 to 89.7) for a low score, 60.4% (53.9 to 66.7) for an intermediate, 38.4% (32.3 to 44.5) for a high and 23.6% (14.0 to 33.2) for a very high score (p < 0.001; Figure 1).
Table 1 presents the multivariable analysis of the developed score considering OS (p < 0.001) and relapse-free survival (RFS, p < 0.001). The score had an concordance index of 0.628 for OS and 0.606 for RFS compared with the TRI (0.554 and 0.562), the IPSS-R (0.562 and 0.559), and the PS (0.569 and 0.562), indicating an improvement in prognostic capability.
Conclusion
Using the EBMT registry, we could validate the MDS transplant risk scores from GITMO and CIBMTR. Furthermore, we identified a positive CMV status of the recipient, MUD, platelet ≤ 25 and > 1% blood blast counts at time of allo-SCT as additional independent transplant-specific predictors of OS. By combining and optimizing GITMO and CIBMTR scores with these factors we could develop an improved EBMT transplant-specific risk score of MDS patients following allo-SCT.
Kroeger: Neovii: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal